Multiscale Defective Interfaces for Realizing Na‐CO2 Batteries With Ultralong Lifespan
October 09, 2024 - Despite their promising high energy density and potential for CO2 recycling, Na‐CO2 batteries face challenges in long-term cycling stability. Researchers from the Technical University of Ilmenau, Ulm University, Zhengzhou University, and the Helmholtz-Zentrum Dresden-Rossendorf have developed a "two-in-one" electrode with multiscale defective interfaces. This advancement extends battery lifespan to over 2400 cycles with improved catalytic activity and stable electrode performance.
Sodium-carbon dioxide (Na-CO2) batteries, which operate based on the conversion reaction 4Na + 3CO2 ↔ 2Na2CO3 + C, feature a high energy density of 1125 Wh kg−1. These batteries offer a dual benefit: recycling the greenhouse gas CO2 and storing electricity generated from renewable energy sources.1 However, despite their potential, the development of Na-CO2 batteries is hindered by inherent limitations, such as their typically short lifespan.1 A critical challenge arises from the dissolution and deposition of the highly reactive sodium metal during repeated energy storage and release cycles. This process can lead to significant volume changes, the formation of dendritic sodium, or the accumulation of inactive “dead” sodium, all of which adversely affect durability and can cause premature battery failure.2 To mitigate these issues, excess sodium foil is often used to compensate for sodium loss. However, this approach not only adds unnecessary material but also fails to address the underlying problem of dendrite formation.3 To date, various strategies have been proposed to address the challenges associated with both electrodes in Na-CO2 batteries. For the anode, approaches such as utilizing solid-state electrolytes have been explored to ensure a stable sodium flow and prevent the formation of sodium dendrites.7 However, most existing studies focus on individual electrodes, which fails to fundamentally resolve the interconnected challenges faced by Na-CO2 systems. A crucial step forward involves systematically organizing both the CO2 cathode and the Na anode, rather than treating them in isolation. By synchronizing their design and operation, the inherent limitations of current Na-CO2 batteries could be effectively addressed. Despite the potential of such an integrated approach, research in this area has yet to deliver significant breakthroughs, leaving it as an ongoing challenge for advanced Na-CO2 systems.
A New Electrode Design for Enhanced Na-CO₂ Battery Performance
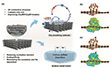
To achieve bidirectional electrodes with highly efficient active sites for both CO2 reduction and evolution reactions (CO2RR/CO2ER) as well as sodium deposition, a “two-in-one” design concept has been proposed. This approach aims to integrate sodiophilic and CO2 redox sites onto the surface of 3D porous host structures. The phenomenon of “atomic size misfit” plays a critical role in this context, as it induces lattice distortions, dislocations, and interfacial defects.4,5 These structural irregularities significantly influence the distribution of lattice stress, surface charges on the electrode, and subsequent interfacial reactions, such as sodium deposition/stripping and CO2 conversion. A thorough understanding of the relationship between defect structures in the host material and battery performance at the atomic level is crucial for optimizing CO2 redox behavior and sodium deposition processes. Researchers from the Technical University of Ilmenau, Ulm University, Zhengzhou University, and the Helmholtz-Zentrum Dresden-Rossendorf have now demonstrated the potential of a bidirectional electrode design that utilizes a 3D structure with consistent ion and electron transport kinetics to enhance the performance and durability of Na-CO2 batteries. This innovative electrode concept fulfills two critical roles. On the anode side, it serves as a sodiophilic host with low nucleation barriers, enabling homogeneous sodium flux and facilitating the migration of Na+ ions throughout the host material for uniform deposition. On the cathode side, the electrode acts as a self-supporting structure with catalytic activity, accelerating the kinetics of CO2 reduction and evolution reactions (CO2RR/CO2ER). To support this concept, the authors performed density-functional theory (DFT) calculations to model electrodes composed of carbon paper-supported Cu-doped iron oxide nanoparticles (CP@FeCu) as well as their single-component counterparts, CP@Fe and CP. A simplified theoretical model of an Fe3O4 cluster doped with a single copper atom was developed to explore the interactions between Fe and Cu. This model highlights how the embedded Cu atom modulates the electronic properties of the Fe3O4 matrix, influencing reaction pathways, energy barriers, and catalytic activity. The charge density differences observed in CP@Fe and CP@FeCu interfaces (Figs. 1b, c) indicate that copper doping significantly enhances charge redistribution. This redistribution facilitates interfacial charge transfer from Fe3O4 clusters to the carbon substrate, pointing to strong interactions at the CP@FeCu interface.
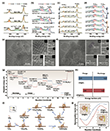
Researchers employed high-resolution transmission electron microscopy (HRTEM) and selected area electron diffraction (SAED) to investigate the structural properties of CP@FeCu at the atomic level. These analyses were conducted using the CC/CS-corrected SALVE microscope at an accelerating voltage of 80 kV.6 The representative HRTEM images reveal densely packed nanoparticles with nanometer-sized grains and a high density of grain boundaries (GBs) in the CP@FeCu matrix (Figures 2a,h). Region I in Figure 2a highlights a twinned structure of Cu nanoparticles, accompanied by lattice fringes with d-spacings of 0.209 nm, corresponding to the (111) plane of the Cu phase. Enlarged images (Figures 2b,c) confirm the presence of Cu atomic defects, as evidenced by variations in line intensity distributions in the selected yellow dashed area. Region II (Figure 2e) shows lattice spacings of 0.243 nm, characteristic of the (222) plane of Fe3O4. Additionally, some nanoparticles exhibit features of twinned structures with a lattice distance of 0.235 nm, which could be attributed to the (222) planes of CuO or Fe3O4 phases (Figure 2i). Single-crystal Cu is also observed, with Cu–Cu atomic distances measured at 2.29 Å (Figure 2l). The authors used inverse fast Fourier-transform (IFFT) analysis to further elucidate these structural features, revealing abundant disordered lattice structures with crystallographic defects and edge dislocations (Figures 2d,f,j,k). Lattice distortion and strain were analyzed using geometric phase analysis (Figures 2a,h), where the shear strain distribution (exy) predominantly propagates along GBs and defect sites (Figures 2g,n). These findings confirm the presence of significant lattice defects in the CP@FeCu matrix. Furthermore, SAED patterns (Figure 2m) indicate the presence of polycrystalline phases, including Fe3O4 and Cu species. Element mapping and line-scan profiles (Figure 2o) demonstrate a uniform distribution of Fe and Cu within the CP@FeCu matrix, mixed with nitrogen and carbon, underscoring the homogeneous composition of the active materials.
Electrochemical Performance and Long-Term Stability of CP@FeCu
The authors examined the sodiophilicity of CP@FeCu by evaluating the voltage-time profiles of different hosts, as shown in Figure 3a. CP@FeCu demonstrated exceptional cycling stability, completing 1980 cycles with an average Coulombic efficiency (CE) of approximately 99.1% (Figure 3b), significantly surpassing other hosts. This performance marks a substantial improvement over conventional sodiophilic coating techniques,7–11 carbon skeletons,12–15 and metallic scaffolds,16,17 as summarized in Figure 3h. The observed disparities highlight the superior ability of sodiophilic CP@FeCu interfaces to stabilize plating and stripping processes during extended cycling. Aurbach Coulombic efficiency tests18 further validated the effectiveness of CP@FeCu, with a CE of 99.3% compared to 98% for CP@Fe and 96.9% for CP. The authors observed that CP exhibited a low activation voltage during the initial pre-activation stage, attributed to the insertion of sodium ions into graphitized carbon fibers. This pre-insertion reduced the nucleation barrier for subsequent sodium plating but consumed a significant amount of Na+, leading to a lower proportion of sodium available for plating and stripping.19
Building on its unique interface structure and favorable composition, CP@FeCu was employed as a stand-alone cathode for Na-CO2 batteries, with CP@Fe serving as a control sample. The electrochemical performance was initially evaluated using cyclic voltammetry (CV) at a scan rate of 0.1 mV s−1 over a voltage range of 1.8–4.2 V (Figure 4a). Peaks observed at approximately 2.3 V and 4 V correspond to the CO2 reduction reaction (CO2RR) and CO2 evolution reaction (CO2ER), respectively. Notably, the CP@FeCu cathode displayed distinct redox peaks, with a reduction peak at approximately 3.17 V and a pronounced oxidation peak at 3.70 V. In contrast, the CP@Fe cathode showed no significant redox activity in this voltage range. These redox peaks at 3.17 V and 3.70 V are likely attributed to valence oscillations of Fe0/Fe2+/Fe3+ and Cu0/Cu2+, indicating that copper doping facilitates charge redistribution between iron and copper species. This redistribution enhances CO2RR activity and plays a critical role in CO2ER, which is essential for lowering the overpotential in Na-CO2 batteries.20–22 As shown in Figure 4b, the CP@FeCu cathode exhibits a charge-discharge voltage gap of 1.55 V, which is significantly lower than the 2.0 V observed for CP@Fe-based batteries, further underscoring its superior electrochemical performance.
The cycling performance of Na-CO2 batteries was further investigated under current densities of 5 and 10 μA cm−2, with a fixed cycle duration of 2 hours (Figures 4c,d). The CP@Fe cathode demonstrated a limited cycle life, achieving approximately 450 hours with 210 stable cycles at 10 μA cm−2. This limitation is attributed to the restricted number of catalytically active sites available for facilitating CO2 reduction (CO2RR) and evolution (CO2ER). As a result, the discharge product Na2CO3 could not be effectively converted during charging, leading to its accumulation. Over time, this accumulation of insulated Na2CO3 hinders Na+ and CO2 transport, impairs charge transfer, and causes a significant increase in polarization, ultimately resulting in battery failure during extended cycling. Remarkably, the CP@FeCu cathode significantly enhances the cycling performance of Na-CO2 batteries, achieving a threefold increase in cycle life compared to CP@Fe. The CP@FeCu-based batteries reached 600 stable cycles at 10 μA cm−2, demonstrating its superior ability to support long-term cycling and maintain stable performance under the same conditions.
As the catalyst undergoes repeated cycling, the oxidation states of Fe and Cu reach a more stable configuration, resulting in a uniform charge–discharge profile. This observation highlights the critical role of valence state oscillations of Fe and Cu in enhancing CO2 reduction (CO2RR) and evolution (CO2ER) kinetics. The stabilization of these redox-active sites contributes to the improved and more consistent performance of the battery over extended cycling. Additionally, the CP@FeCu cathode enables the battery to achieve stable operation at a current density of 5 μA cm−2 for 2400 cycles (4800 hours), maintaining a high discharge voltage of 2.2 V, which significantly outperforms the CP@Fe cathode. These results demonstrate that Cu doping effectively adjusts the electronic structure of CP@Fe, promoting CO2 activation and Na2CO3 nucleation during discharge while simultaneously lowering the decomposition barrier of Na2CO3 during charging. This leads to improved redox kinetics, reduced polarization, and enhanced cycling stability. Notably, the exceptional longevity of the CP@FeCu-based battery, as shown in Figure 4e, significantly exceeds previously reported findings on cycling stability.2,3,23
Structural and Chemical Analysis of Discharge and Charge Processes
To gain deeper insights into the reversible CO2 reduction (CO2RR) and evolution (CO2ER) processes on the CP@FeCu cathode, ex situ characterizations including XRD, Raman spectroscopy, and X-ray photoelectron spectroscopy (XPS) were conducted to analyze the evolution of discharge products. Significant changes in the XRD and Raman patterns (Figures 4f,g) of the discharged CP@FeCu cathode reveal the formation of Na2CO3 crystals as the primary discharge products. Upon recharging, all signals associated with Na2CO3 disappear, indicating its complete decomposition. TEM analysis (Figure 4h) shows that the CP@FeCu cathode surface is densely covered with discharge products, while the corresponding SAED spectra (Figure 4i) confirm the presence of Na2CO3 with diffraction patterns indexed to (201), (310), (221), (−512), and (313) planes. Additional diffraction patterns (111) and (311) are attributed to copper in CP@FeCu. HAADF-STEM imaging and EDS mapping, along with line-scan profiles, further substantiate that the predominant discharge product is Na2CO3 with a bulk morphology. Upon charging, the cathode surface becomes distinctly clear (Figure 4j), and only the diffraction patterns of copper are observed (Figure 4k), confirming the complete decomposition of Na2CO3.
The evolution of discharge products is further evidenced by SEM images. During discharge, Na2CO3 uniformly nucleates and covers the entire carbon fiber surface (Figure 4l). During the charging process, Na2CO3 undergoes gradual decomposition, eventually re-exposing the carbon fiber surface (Figure 4m). These results strongly support the conclusion that CP@FeCu exhibits highly efficient electrocatalytic activity, facilitating the reversible formation and decomposition of Na2CO3 and enabling excellent rechargeability in Na-CO2 batteries. The pristine Cu 2p spectrum (Figure 5a, bottom) reveals main peaks at 932.2 eV and 952.1 eV, corresponding to the Cu(0) phase. Additionally, peaks at 934.7 eV and 954.5 eV, along with pronounced satellite peaks at 940.1 eV and 943.6 eV, indicate the presence of the Cu(II) phase.24 The gradual disappearance of characteristic Cu(II) peaks with increasing etching time suggests that the Cu(II) phase is primarily located on the surface of the CP@FeCu substrate. The pristine Fe 2p spectrum (Figure 5b, bottom) displays two pairs of peaks for Fe 2p3/2 and Fe 2p1/2, identified as Fe(II) (710.0 and 723.6 eV) and Fe(III) (712.2 and 727.2 eV).20 Quantitative analysis of the Fe(II)/Fe(III) ratio through peak fitting suggests approximate equivalence. The emergence of a peak at 707.5 eV associated with Fe(0) during etching indicates a small amount of Fe(0) phase within the bulk of the CP@FeCu substrate. Following the discharge and charging processes (Figures 5c,d), the disappearance of the Fe(0) signal and a significant enhancement of Fe3+ and Cu2+ signals were observed. These changes highlight the critical role of charge redistribution between Fe and Cu in promoting CO2 reduction (CO2RR) and evolution (CO2ER). Quantitative analysis further indicates that the redox states of Fe and Cu oscillate from the surface to the bulk during discharge and charging, suggesting that these valence oscillations are key to the high CO2RR and CO2ER activity observed. The multiscale defect structures in CP@FeCu samples after discharge and charging states were also examined, providing further insight into the material's structural dynamics.
Defect Engineering and Its Impact on Catalytic Performance
High-resolution transmission electron microscopy (HRTEM) images (Figures 5e,f) reveal that the interconnected nanoparticle structure of CP@FeCu, characterized by abundant grain boundaries, consists of numerous crystalline-amorphous nanodomains. Enlarged HRTEM images and corresponding inverse fast Fourier transform (IFFT) images confirm that the discharged and charged CP@FeCu samples retain defective structural features, including Cu atom defects, edge dislocations, and distinctly distorted atomic arrangements. Notably, Cu atoms in Cu-rich regions are observed to penetrate and extend into the lattice of Fe3O4 grains, as shown in the enlarged images of Region I (Figure 5e) and Region VI (Figure 5f). This penetration induces complex lattice distortions and nanostructural defects, which enhance electron distribution dynamics and oxidation state interactions between the defective Fe─Cu active sites, thereby promoting the catalytic processes of CO2 reduction (CO2RR) and evolution (CO2ER). The well-preserved defect structures observed after multiple charge and discharge cycles highlight the structural durability and functional stability of CP@FeCu, ensuring excellent cycling stability in prolonged battery operation. To better understand the intrinsic relationship between the optimized electronic structure of the CP@FeCu interface and its enhanced catalytic activity in Na-CO2 conversion, the Gibbs free energy evolution for each step of the charge/discharge process at U = 0 V was calculated (Figure 5g). Following a four-electron reaction pathway,25,26 it is generally assumed that CO2ER is the reverse of CO2RR, suggesting that both reactions share identical active sites and reaction intermediates. Figure 5i illustrates potential reaction pathways at the CP@FeCu and CP@Fe interfaces, along with optimized structures of intermediates and transition states, providing valuable insights into the catalytic mechanisms.
As shown in Figure 5h, the discharge process exhibits a maximum free energy barrier (ΔGmax) of 6.27 eV for the CP@Fe interface and 5.47 eV for the CP@FeCu interface. During the charging process, ΔGmax values of 4.41 eV and 3.02 eV were observed for CP@Fe and CP@FeCu, respectively. These results indicate that both CO2 reduction (CO2RR) and CO2 evolution (CO2ER) are thermodynamically more favorable on the CP@FeCu interface, owing to the significantly lower free energy barriers compared to the CP@Fe interface. Initial theoretical analyses further reveal that charge redistribution between Fe and Cu atoms weakens the interactions within Na─O bonds, facilitating the decomposition of Na2CO3. The decomposition barriers of Na2CO3 at the two interfaces were also calculated (Figure 5j), showing that the CP@FeCu interface exhibits a significantly reduced decomposition barrier compared to the CP@Fe surface. This reduction enhances the efficiency of active site utilization, minimizes the formation of inactive Na2CO3, and lowers the charging voltage. These computational findings align closely with experimental observations, underscoring the superior catalytic performance of the CP@FeCu interface. A comparative analysis was performed using a full-cell configuration comprising a CP@FeCu-Na anode and a CP@FeCu cathode (CP@FeCu-Na||CP@FeCu) (Figure 6a). As shown in Figures 6b,c, the CP@FeCu-Na electrode exhibits a dense and smooth deposition morphology with a total electrode thickness of approximately 0.45 mm and a sodium loading of 50 mAh cm−2. This structure ensures uniform sodium deposition, which is critical for maintaining stable cell performance.
In Figure 6d, the terminal discharge voltage of the CP@FeCu-Na||CP@FeCu cell remains above 2 V even after 100 cycles, whereas the Cu-Na||CP@FeCu cell experiences a rapid decline in voltage after only 20 cycles. Furthermore, the CP@FeCu-Na||CP@FeCu cells demonstrate remarkable cycling stability, enduring over 240 cycles (480 hours) at 5 μA cm−2 and surpassing 100 cycles (200 hours) at 10 μA cm−2, all while maintaining nearly constant charge/discharge profiles (Figure 6e). These findings underscore the superior performance and durability of the CP@FeCu-Na||CP@FeCu configuration in comparison to conventional systems. The new work demonstrates a significant advancement in Na-CO2 battery technology, with the CP@FeCu-based system extending the battery lifespan to over 2400 cycles. The reduced energy barriers for sodium deposition and Na2CO3 decomposition contribute to lower polarization and improved charge/discharge efficiency. These findings highlight the potential of CP@FeCu electrodes as a promising platform for the development of next-generation energy storage systems that combine high performance, durability, and the ability to recycle CO2.
Resource: Xu, C., Hong, P., Dong, Y., Li, Y., Shen, Y., Biskupek, J., Zhao, H., Kaiser, U., Shao, G., Lei, Y. Multiscale Defective Interfaces for Realizing Na-CO2 Batteries With Ultralong Lifespan. Adv. Mater. 2024, 36, 2409533. 10.1002/adma.202409533
-
Xu, C., Dong, Y., Shen, Y., Zhao, H., Li, L., Shao, G., & Lei, Y. (2023). Fundamental understanding of nonaqueous and hybrid Na–CO2 batteries: challenges and perspectives. Small, 19(15), 2206445.
-
Mao, Y., et al. (2022). Forging inspired processing of sodium‐fluorinated graphene composite as dendrite‐free anode for long‐life Na–CO2 cells. Energy & Environmental Materials, 5(2), 572-581.
-
Chen, X., et al. (2023). Advanced Li/Na–CO2 batteries enabled by the γ-MnO2 catalyst with enhanced carbonate decomposition. ACS Applied Materials & Interfaces, 15(23), 28106-28115.
-
Francis, M. F., Neurock, M. N., Zhou, X. W., Quan, J. J., Wadley, H. N. G., & Webb, E. B. (2008). Atomic assembly of Cu/Ta multilayers: Surface roughness, grain structure, misfit dislocations, and amorphization. Journal of Applied Physics, 104(3).
-
Zhang, Q., et al. (2024). In situ and real‐time monitoring the chemical and thermal evolution of lithium‐ion batteries with single‐crystalline Ni‐rich layered oxide cathode. Angewandte Chemie, 136(18), e202401716.
-
Linck, M., et al. (2016). Chromatic aberration correction for atomic resolution TEM imaging from 20 to 80 kV. Physical Review Letters, 117(7), 076101.
-
Xie, Y., et al. (2020). Encapsulating sodium deposition into carbon rhombic dodecahedron guided by sodiophilic sites for dendrite-free Na metal batteries. Energy Storage Materials, 30, 1-8.
-
Xiao, H., et al. (2023). Stabilize sodium metal anode by integrated patterning of laser‐induced graphene with regulated Na deposition behavior. Small, 19(47), 2303959.
-
Zhuang, R., et al. (2023). Fluorinated porous frameworks enable robust anode-less sodium metal batteries. Science Advances, 9(39), eadh8060.
-
Liu, P., Wang, X., Jia, X., & Zhou, J. (2022). Carbon-confined two-dimensional sodiophilic sites boosted dendrite-free sodium metal anodes. ACS Applied Materials & Interfaces, 14(31), 35873-35882.
-
Yuan, R., Liu, P., Wang, X., & Zhou, J. (2023). Interlayer sodium plating/stripping in van der Waals‐layered quantum dot superstructure. Small, 19(28), 2300919.
-
Xiao, J., et al. (2022). Sodium metal anodes with self‐correction function based on fluorine‐superdoped CNTs/cellulose nanofibrils composite paper. Advanced Functional Materials, 32(21), 2111133.
-
Xu, M., et al. (2023). Uniform SnSe nanoparticles on 3D graphene host enabling a dual-nucleation-site interface for dendrite-free sodium metal batteries. Energy Storage Materials, 60, 102848.
-
Xu, Z., et al. (2021). Homogenous metallic deposition regulated by defect-rich skeletons for sodium metal batteries. Energy & Environmental Science, 14(12), 6381-6393.
-
Xu, C., et al. (2024). Dual‐functional electrode promoting dendrite‐free and CO2 utilization enabled high‐reversible symmetric Na‐CO2 batteries. Energy & Environmental Materials, 7(3), e12626.
-
Yang, C., Zhang, Y., Hua, Y., Wang, H., Tang, A., & Yang, H. (2023). Functionalized Halloysite scaffold controls sodium dendrite growth. ACS Applied Materials & Interfaces, 15(9), 11949-11960.
-
Yang, W., Yang, W., Dong, L., Shao, G., Wang, G., & Peng, X. (2021). Hierarchical ZnO nanorod arrays grown on copper foam as an advanced three-dimensional skeleton for dendrite-free sodium metal anodes. Nano Energy, 80, 105563.
-
Adams, B. D., Zheng, J., Ren, X., Xu, W., & Zhang, J. G. (2018). Accurate determination of coulombic efficiency for lithium metal anodes and lithium metal batteries. Advanced Energy Materials, 8(7), 1702097.
-
Zhuang, R., et al. (2023). Fluorinated porous frameworks enable robust anode-less sodium metal batteries. Science Advances, 9(39), eadh8060.
-
Xu, C., Zhan, J., Wang, H., Kang, Y., & Liang, F. (2021). Dense binary Fe–Cu sites promoting CO2 utilization enable highly reversible hybrid Na–CO2 batteries. Journal of Materials Chemistry A, 9(38), 22114-22128.
-
Xu, C., et al. (2021). Biomass-derived highly dispersed Co/Co9S8 nanoparticles encapsulated in S, N-co-doped hierarchically porous carbon as an efficient catalyst for hybrid Na–CO2 batteries. Materials Today Energy, 19, 100594.
-
Xu, C., Wang, H., Zhan, J., Kang, Y., & Liang, F. (2022). Engineering NH3-induced 1D self-assembly architecture with conductive polymer for advanced hybrid Na–CO2 batteries via morphology modulation. Journal of Power Sources, 520, 230909.
-
Xu, B., et al. (2022). Fabrication of long-life quasi-solid-state Na–CO2 battery by formation of Na2C2O4 discharge product. Cell Reports Physical Science, 3(7).
-
Guan, A., et al. (2020). Boosting CO2 electroreduction to CH4 via tuning neighboring single-copper sites. ACS Energy Letters, 5(4), 1044-1053.
-
Tan, C., Wang, A., Cao, D., Yu, F., Wu, Y., He, X., & Chen, Y. (2023). Unravelling the complex Na2CO3 electrochemical process in rechargeable Na‐CO2 batteries. Advanced Energy Materials, 13(13), 2204191.
-
Xu, C., et al. (2020). Reversible hybrid sodium-CO2 batteries with low charging voltage and long-life. Nano Energy, 68, 104318.