A novel low-dimensional polymer with potential for electrical anisotropy
January 12, 2025 - Researchers have synthesized a novel electrically conductive coordination polymer (ECCP), Cu5BHT, exhibiting a low-symmetry structure and in-plane electrical anisotropy. Using a water-surface synthesis method, they achieved precise control over the formation of interconnected CuS4 and Cu2S4 units, which influence the material's charge transport properties. High-resolution electron microscopy and diffraction techniques confirmed the structural asymmetry, while theoretical calculations and single-crystal electrical measurements demonstrated its metallic nature. Notably, Cu5BHT shows significant anisotropic conductivity, with an anisotropic factor of approximately 8. These findings provide a new pathway for developing low-symmetry ECCPs with tunable electrical properties for advanced nanotechnology applications.
Electrically conductive coordination polymers (ECCPs) and two-dimensional conjugated metal-organic frameworks (c-MOFs), distinguished by intralayer π-d conjugation and interlayer electronic coupling, have attracted considerable interest due to their diverse electronic properties, ranging from semiconducting to metallic behavior.1,2 The controlled bottom-up synthesis of ECCPs allows for precise tailoring of electronic structures at the molecular level, positioning these materials as promising candidates for next-generation electronic and quantum applications.3 Recent advancements in the field have led to a rapid expansion of the ECCP family, with a particular focus on the design of periodic networks featuring square, honeycomb, and Kagome lattice architectures. These structures are typically assembled through square-planar MX4 linkages (where M represents a metal ion and X corresponds to O, NH, or S) in combination with conjugated organic ligands such as benzenehexathiol (BHT),1,3 hexahydroxybenzene (HHB),4 and hexaiminotriphenylene (HITP).5
Among electrically conductive coordination polymers, nonporous ECCPs based on benzene-derived building units, such as CuxBHT (x = 3, 4, 5.5),1,3 Cu3HHB,4 and Cu4DHTTB (DHTTB = 2,5-dihydroxy-1,3,4,6-tetrathiolbenzene),6 have attracted particular interest due to their tunable structural topology, diverse composition, and exceptional electronic properties. Compared to other ECCPs, these materials adopt a Kagome lattice with a high density of ligands and metal ions, which facilitates extended π-electron conjugation and strong π-d orbital interactions. This structural arrangement results in well-dispersed electronic bands and narrow—or even zero—band gaps.4 Despite notable advancements in the development of ECCPs with enhanced electronic conductivity and charge carrier mobility over the past decade, the study of their anisotropic charge transport properties remains ongoing.4,7–10 To date, the experimental realization of low-symmetry ECCPs exhibiting in-plane electrical anisotropy has yet to be demonstrated.
Synthesis and Structural Characterization of Cu5BHT
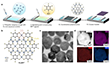
Figure 1a illustrates the synthesis process of Cu5BHT (Figure 1b) on the water surface, which proceeds in three distinct steps.
First, a Langmuir trough was filled with an aqueous Cu(NO3)2 solution (~2 × 10−4 M), serving as the subphase.
Next, the authors used 150 μL of a freshly prepared solution of benzenehexathiol (BHT) (0.3 mg mL−1) in a chloroform/N,N-dimethylformamide (DMF) mixture (2:1 v/v), which was carefully spread onto the water surface to ensure uniform distribution.
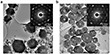
After 10 hours of polymerization (Step III), a black film with a lateral size of approximately 50 cm2 formed on the water surface, visible to the naked eye. Using the horizontal dipping method, the authors successfully transferred the film onto various substrates, including Si/SiO2 and copper grids, to facilitate further morphological and structural analysis. Transmission electron microscopy (TEM) images revealed that the film comprises densely packed crystal domains, interspersed with smaller crystallites and an amorphous thin film (Figure 2). Notably, the primary crystal domains exhibit an asymmetric hexagonal morphology, with lateral dimensions ranging from 200 nm to 1 μm (Figure 1b). Further elemental analysis using scanning transmission electron microscopy energy-dispersive X-ray (STEM-EDX) mapping confirmed a uniform distribution of carbon, sulfur, and copper atoms across the entire domain (Figure 1c).
Crystallographic Analysis and Phase Purity
Imaging and diffraction techniques were employed to investigate the crystal structure of the synthesized Cu5BHT. A low-magnification TEM image reveals an asymmetric hexagonal Cu5BHT flake (Figure 3a), while selected-area electron diffraction (SAED) recorded perpendicularly to the crystal surface confirms a hexagonal asymmetry with sharp diffraction spots, indicating a single-crystalline nature (Figure 3b).
The presence of unequal angles (≠ 120°) between different zone axes suggests a low-symmetry crystal lattice. First-order diffraction spots were observed at 1.35 nm–1, corresponding to a d-spacing of 7.4 Å.
To achieve higher spatial resolution while minimizing electron radiation damage, the authors performed low-dose aberration-corrected high-resolution TEM (AC-HRTEM) at an accelerating voltage of 80 kV, using a dose rate of 3.2 × 103 e– Å–2s–1.
As shown in Figure 3c, the AC-HRTEM image with a resolution of 0.77 Å reveals well-aligned Kagome-like lattices, free from noticeable structural defects or domain boundaries, in excellent agreement with the crystal structure of Cu5BHT. The measured lattice distance of 7.4 Å is consistent with the values obtained from SAED patterns. In the enlarged image, individual atomic positions are clearly resolved, highlighting an asymmetric structure composed of two distinct types of secondary building units (SBUs): square planar CuS4 and non-planar Cu2S4, as evidenced by the varying contrast of Cu atoms (Figure 3d and 3e). The scientists found that Cu2S4 is arranged in layers extending along the (110) and (1-10) crystal directions, interconnected by chains of alternating benzene rings and CuS4 SBUs aligned along the (010) direction. Furthermore, fast Fourier transform (FFT) analysis of the AC-HRTEM images revealed a six-fold asymmetry that closely matched the SAED pattern, demonstrating that the longest diagonal in the crystal corresponds to the (010) direction. This diagonal aligns with the two angles measuring less than 120° (Figure 4).
As shown in Figure 3f, the grazing-incidence wide-angle X-ray scattering (GIWAXS) pattern exhibits discrete Bragg spots near Qz = 0, indicating high crystallinity on a macroscopic scale. The in-plane peaks at Qxy = 0.86, 1.49, 1.72, and 2.56 Å–1 correspond to the (200), (310), (400), and (600) Bragg reflections, respectively, with a d-spacing of 7.3 Å (Figure 3g). These findings are consistent with the results obtained from AC-HRTEM and SAED analyses. An intense arc at 1.82 Å–1 suggests an interlayer stacking distance of ~3.45 Å, confirming a preferential face-on orientation. The data further indicate that Cu5BHT crystallizes in the orthorhombic P2gg space group, with unit cell parameters of a = 14.69 Å, b = 8.48 Å, and c = 3.45 Å. To validate these structural characteristics, the scientists compared the AC-HRTEM image, SAED pattern, and projection profile with simulated data (Figure 3e), finding excellent agreement that confirms the phase purity of Cu5BHT. Based on these experimental results, a high-quality atomic model of Cu5BHT was constructed for density functional theory (DFT) calculations, revealing a distinct local structure with low symmetry (Figure 3h).
Chemical Stability and Charge Transfer Mechanism
The reduction of Cu2+ is likely driven by electron transfer from sulfur. The formation of Cu5BHT occurs in three successive steps: first, Cu2+ reacts with a thiol to form a Cu2+-thiol complex (Step 1). This is followed by electron transfer from the thiol, leading to the formation of Cu+ and a thiol radical (Step 2).11,12 Finally, Cu+ reacts with a second thiol, resulting in the formation of a (Cu+)2 cluster-thiol or Cu+-thiol complex (Step 3).
Peak deconvolution of the S 2s spectrum reveals that CuS4 and Cu2S4 moieties exhibit oxidation states of –1 and 0 in a 31:69 ratio,13 consistent with the CuS4:Cu2S4 ratio of 1:2.
TEM and SAED analyses confirm that Cu5BHT crystals retain their structural integrity and crystallinity even after solvent washing and annealing, underscoring their remarkable chemical and thermal stability (Figure 5).
Extended X-ray absorption fine structure (EXAFS) spectra of Cu5BHT (Figures 6b,c) provide detailed insights into the coordination environment of Cu+-S at 1.85 Å, while a second peak at 2.31 Å reflects the contribution of Cu-Cu interactions within the Cu2S4 structural unit.14
Electronic Properties and In-Plane Anisotropic Conductivity
To investigate the electrical properties of Cu5BHT, the authors fabricated devices by establishing electrical connections between the crystals and Cr/Au electrodes using helium ion beam deposition of Pt wires (Figure 7a). This approach, which offers higher resolution and reduced sample damage compared to conventional gallium ion beam sources, enabled precise contact formation.
Electrical characterization confirmed that these connections exhibit Ohmic behavior, as evidenced by the linear 4-point-probe (4pp) I-V characteristics (Figure 7b). The consistency of electrical contacts at all six electrode-ECCP interfaces further supports the high-quality connection.
Temperature-dependent electrical measurements were conducted in the range of 300 to 30 K. As shown in Figure 7c, conductance increased monotonically along all crystal directions—(010), (110), and (1-10)—upon cooling, a hallmark of metallic behavior.
At room temperature (Figure 7c, inset), the measured conductance along the (010), (110), and (1-10) crystal directions revealed a significant anisotropic factor, A = σ(010)/σ(110) or σ(010)/σ(1-10), of approximately 8.
This trend, where σ(010) > σ(110) ≈ σ(1-10), aligns well with theoretical predictions and provides the first experimental demonstration of in-plane electrical anisotropy in metallic Cu5BHT. Notably, this behavior surpasses that of other CuxBHT phases (x = 3, 4, 5.5), for which comparable anisotropy has not been observed at the device level.
Resource: Wang, Z., Un, H.-I., Liu, T.-J., Liang, B., Polozij, M., Hambsch, M., Pöhls, J. F., Weitz, R. T., Mannsfeld, S. C. B., Kaiser, U., Heine, T., Sirringhaus, H., Feng, X., & Dong, R. (2025). A Low-Symmetry Copper Benzenehexathiol Coordination Polymer with In-Plane Electrical Anisotropy. Angewandte Chemie International Edition, 2025, e202423341. https://doi.org/10.1002/anie.202423341
-
Huang, X., Sheng, P., Tu, Z., Zhang, F., Wang, J., Geng, H., et al. (2015). A two-dimensional π–d conjugated coordination polymer with extremely high electrical conductivity and ambipolar transport behavior. Nature Communications, 6(1), 7408.
-
Dou, J. H., Sun, L., Ge, Y., Li, W., Hendon, C. H., Li, J., et al. (2017). Signature of metallic behavior in the metal–organic frameworks M3 (hexaiminobenzene)2 (M= Ni, Cu). Journal of the American Chemical Society, 139(39), 13608-13611.
-
Huang, X., Qiu, Y., Wang, Y., Liu, L., Wu, X., Liang, Y., et al. (2020). Highly conducting organic–inorganic hybrid copper sulfides CuxC6S6 (x = 4 or 5.5): ligand‐based oxidation‐induced chemical and electronic structure modulation. Angewandte Chemie, 132(50), 22791-22798.
-
Wang, Z., St. Petkov, P., Zhang, J., Liang, B., Revuelta, S., Xiao, K., et al. (2024). Benzenehexol‐based 2D conjugated metal–organic frameworks with Kagome lattice exhibiting a metallic state. Advanced Functional Materials, 2404680.
-
Chen, T., Dou, J. H., Yang, L., Sun, C., Libretto, N. J., Skorupskii, G., et al. (2020). Continuous electrical conductivity variation in M3 (hexaiminotriphenylene)2 (M= Co, Ni, Cu) MOF alloys. Journal of the American Chemical Society, 142(28), 12367-12373.
-
Huang, X., Fu, S., Lin, C., Lu, Y., Wang, M., Zhang, P., et al. (2023). Semiconducting Conjugated Coordination Polymer with High Charge Mobility Enabled by “4 + 2” Phenyl Ligands. Journal of the American Chemical Society, 145(4), 2430-2438.
-
Dou, J. H., Arguilla, M. Q., Luo, Y., Li, J., Zhang, W., Sun, L., et al. (2021). Atomically precise single-crystal structures of electrically conducting 2D metal–organic frameworks. Nature Materials, 20(2), 222-228.
-
Day, R. W., Bediako, D. K., Rezaee, M., Parent, L. R., Skorupskii, G., Arguilla, M. Q., et al. (2019). Single crystals of electrically conductive two-dimensional metal–organic frameworks: Structural and electrical transport properties. ACS Central Science, 5(12), 1959-1964.
-
Wang, Z., Walter, L. S., Wang, M., Petkov, P. S., Liang, B., Qi, H., et al. (2021). Interfacial synthesis of layer-oriented 2D conjugated metal–organic framework films toward directional charge transport. Journal of the American Chemical Society, 143(34), 13624-13632.
-
Liu, E., Fu, Y., Wang, Y., Feng, Y., Liu, H., Wan, X., et al. (2015). Integrated digital inverters based on two-dimensional anisotropic ReS2 field-effect transistors. Nature Communications, 6(1), 1-7.
-
Brust, M., Blass, P. M., & Bard, A. J. (1997). Self-assembly of photoluminescent copper (I)− dithiol multilayer thin films and bulk materials. Langmuir, 13(21), 5602-5607.
-
Daniel, T. A., Uppili, S., McCarty, G., & Allara, D. L. (2007). Effects of molecular structure and interfacial ligation on the precision of Cu-bound α, ω-mercaptoalkanoic acid “molecular ruler” stacks. Langmuir, 23(2), 638-648.
-
Karthikeyan, M., Bhagyaraju, B., Mariappan, C. R., Mobin, S. M., & Manimaran, B. (2012). Novel semiconducting metal-organic framework: Synthesis, structural characterisation and electrical conductivity studies of manganese based two dimensional coordination polymers. Inorganic Chemistry Communications, 20, 269-272.
-
Lin, S. C., Chang, C. C., Chiu, S. Y., Pai, H. T., Liao, T. Y., Hsu, C. S., et al. (2020). Operando time-resolved X-ray absorption spectroscopy reveals the chemical nature enabling highly selective CO2 reduction. Nature Communications, 11(1), 3525.