Formation of crystals filmed in atomic detail
August 28, 2020 - A new study of scientists from Germany, UK and Japan sheds light on the fundamental phenomenon of nucleation and crystal growth. The direct observation of the entire process of the nucleation of 3 different metals was reported with atomic resolution for the first time.
A wide variety of materials are made up of crystals. The classical nucleation theory describes the formation of crystals in a single step. Atoms, ions or molecules crystallize by attaching to an ordered structure (Fig. 1a).[1, 2] According to alternative theories, the nucleation proceeds as a two-step nucleation mechanism (TSNM, Fig. 1b),[3-5] in which an amorphous phase is formed first, which is transformed into a crystalline phase in a second step.[6-8] It is thereby possible that the intermediate phase only changes into the end phase when a certain minimum cluster size is reached, as illustrated in Fig. 1c.[9-10]
Very often, only analysis techniques with a low temporal resolution such as cryo-TEM, [11, 12] X-ray diffraction, [13] atomic force microscopy[1-14] or optical microscopy[15] could be successfully employed to investigate the nucleation steps. Recently, the liquid cell TEM technology made it possible to investigate nucleation and growth processes of metal nanocrystals and inorganic compounds in solutions in real time with high temporal and spatial resolution but the very early stages of nucleation could not be analyzed until now. However, this is where important answers to critical questions about nucleation are still persisting. Therefore, a conceptually new experimental approach is required to enable the study of these early stages of nucleation where the cluster sizes are in the range of <1,000 atoms.[16]
Nano test tubes and electron beams
In their publication in Nature Chemistry, the scientists reported about the in situ TEM (AC-HRTEM) data,[17-19] which they combined with in situ STEM (HAADF-STEM and corresponding EELS) at a voltage of 60 keV to study the early stages of nucleation. They used single-walled carbon nanotubes (SWNT) as an electron-transparent test tubes to stabilize the clusters. In order to keep the transformations caused by the electron beam at an observable level, an acceleration voltage of 80/60 keV was used for HRTEM/STEM observation, below the knock-on threshold of SWNT. They examined three different metals, γ-Fe, Au and Re. The TSNM model was for all three metals proven to be correct under the experimental conditions.[8]
Stage of γ-Fe crystallite nucleation
A constant source of energy is made available to the atoms, the SWNT wall serves as a substrate for a nucleation nucleus (Fig. 2).
0 s: The first stage shows the formation of a “diatomic nucleus”: Two Fe atoms form a pair on the outside of the SWNT wall with a distance of 0.32–0.34 nm between the metal atoms. This distance is significantly longer than the calculated equilibrium bond length of 0.20 nm for an isolated Fe2 molecule and corresponds to a reduction in the binding energy of the dimer from 2.2 eV (at 0.20 nm) to 0.8 eV (at 0.33 nm). This shows the importance of the underlying SWNT substrate in controlling the Fe-Fe distance and makes it more comparable to γ-Fe (0.29 nm) than Fe2 in the gas phase.
8 s: The right Fe atom passes the carbon wall and enters the cavity of the SWNT (Fig. 3c). Since atoms cannot penetrate the carbon lattice of a SWNT, the observed migration of Fe indicates the presence of vacancy defects in the SWNT wall that can facilitate the bonding of the Fe atoms to the SWNT.
13 s: The atomic injector moves along the nanotube and moves in the direction of the diatomic nucleus (Fig. 3a). The consequence of the interaction between the mobile atom injector and the stationary nucleus becomes clear in the next sequence of events (Fig. 3b, c), in which the right Fe atom of the nucleus is pushed out and the distance between them is increased to 0.43 nm.
19 s: The separation frees up space for a third atom, which then moves from the atomic injector into the nucleus. This means the beginning of the second stage of nucleation - the process of atom donation (13–50 s). In the next 4 s, the diatomic nucleus grows into a cluster of 17 Fe atoms, with only one Fe atom remaining in the atomic injector. The structure of the growing Fe cluster remains amorphous at this stage.
23 s: Metallic bonds can act as an attractive force by pulling the atomic injector towards the Fe nucleus and holding it in place during the atomic release stage for injecting most of the Fe atoms (Fig. 3a) The real-time imaging of the atomic release process enables the visualization how the discrete atoms are gradually joined together to become a cluster with the diatomic nucleus.
50 s: At the point of departure of the atomic injector, a stage of the “metastable amorphous state” begins, in which the structureless subnanometer cluster with ~ 17 Fe atoms is continuously reorganized for the next 147 s. During this phase, the Fe atoms oscillate back and forth between the outer and inner surfaces of the SWNT, with the distances between the atoms and the coordination numbers of the Fe atoms changing continuously and randomly over time (Fig. 4a). The highly dynamic nature of the metal cluster at this stage is consistent with the idea of a metastable amorphous cluster where the positions of the atomic constituents are extremely sensitive to local conditions such as temperature, pressure and the local environment. These influence the reorganization process and thus the final structure of the crystallite core.
197 s: Finally, the amorphous cluster is crystallizing (Fig. 4a), whereby the Fe atoms are evenly spaced, which correspond to the (111) plane of the γ-Fe crystal lattice. This represents the fourth stage, which is referred to as "ordered crystallite" and marks the end of the nucleation process, since from this point on the further growth of the crystallite is strictly determined by the structure of the ordered crystal-like core. The analysis of the Fourier transform (FFT) shows a change in the relative degree of crystallinity over time (Fig. 4b).
The existence of the initially amorphous clusters is a key to understanding the underlying mechanism of the nucleation processes. Two-step nucleation mechanisms are much more complex compared to classical nucleation theory, with both their specific details and the question of whether or not they are required to explain nucleation in a variety of different materials being widely discussed scientifically. The scientists were now able to fundamentally investigate the diatomic nucleation stage.
In the diatomic nucleation stage (Fig. 4c), interactions between the diatomic Fe nucleus and the SWNT reduce the total free energy of the dimer and stabilize the nucleus, similar to the stabilization that occurs in heterogeneous nucleation processes such as the gas-solid, the gas-liquid or the solid-liquid interface. However, the diatomic nucleus can dissolve again into discrete atoms, which highlights the metastable nature of the nucleus. When the cluster reached a size of ~ 10 Fe atoms it was not anymore dissociate into individual atoms. The size of the cluster was then increasing to 17-atoms. Importantly, the cluster remained amorphous for 142 s before it crystallizes, evidencing that a metastable amorphous precursor cluster is required for γ-Fe nucleation.
Under the experimental conditions, more than 10 Fe atoms were necessary and 17 Fe atoms were sufficient to produce an ordered crystalline cluster. The exact size might of course depend on the exact experimental conditions.[20-21] When the γ-Fe crystallite is continuously irradiated by the electron beam, it begins to interact with the host SWNT, which led to the removal of carbon atoms from the SWNT and finally to the Rupture of the SWNT after 170 s.[17]
Au crystals
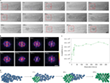
The study of a larger amorphous Au cluster is shown in Figure 5.
0 s: At the beginning of the experiment, an initially stable amorphous Au cluster can be observed, which is enclosed in the SWNT. Some Au atoms diffuse into the neighboring carbon nanostructure (violet arrow, Fig. 5a). In the first 16 s of electron beam irradiation, the atomic structure of the cluster is extremely dynamic and appears amorphous.
17 s: A metastable crystallite with a diameter of less than 1 nm can be observed in the Au cluster (red arrow, Fig. 5a), which is confirmed by analyzing the corresponding FFT pattern (Fig. 5b). This tiny crystallite dissociates in the following 5 s and reappears after 23 s.
23 s: When the diameter of the crystallite reaches ~1 nm, it becomes stable for longer periods of time, whereby a well-ordered structure of the (111) face of Au is observed. From this point on, the nucleation process gradually extends from the tiny crystallite to the entire Au cluster over the following 348 s, reaching a final crystallite size that is ~ 2 nm long and ~ 1 nm wide at 370 s. The Au crystallite maintains its orientation in the first 28 s of the nucleation process from 23 s to 51 s and then rotates or transforms.
51 – 330 s: Different orientations are observed.
331 – 370 s: The last phase of nucleation is observed. The Au crystallite rotates or transforms itself back into the original (111) surface, as can be seen from the FFT patterns of the areas marked with a red frame (Fig. 5a, b). By evaluating the intensity of the FFT pattern, the degree of crystallinity can be estimated (Fig. 5c). A sudden increase indicates heterogeneous nucleation of Au, which occurs via an amorphous precursor, similar as for γ-Fe.
The environmental conditions are supposed to have a great influence on the nucleation. In the case of Au, the metastable amorphous cluster changes the atomic structure dynamically before it finally crystallizes under the influence of the electron beam (Fig. 5d). An Au crystallite has already been found with a radius of ~ 0.45 nm, which is much smaller than the 2 nm crystal nucleus found in TEM liquid cells.[20] Previous work has shown that the energy transferred by the electron beam can affect, melt, and even vaporize the various metal nanocrystals.[22] Therefore, the maximum kinetic energy of the electrons was limited to 80 keV or 0.96 eV per Au atom. This energy transfer seems to be just enough for the cluster to overcome the energy barrier for the amorphous to crystalline transition.
Re crystals
An increasing number of atoms in the cluster is needed to promote the nucleation process. Therefore, the coalescence of clusters can be an important process for nucleation as it allows for a rapid increase in cluster size. In the case of Re, two smaller clusters of ~ 10 atoms collided and merged into a particle large enough to crystallize.
0 s: At the beginning of the TEM time series, there are two existing amorphous Re-clusters in the sub-nanometer range with ~ 10 atoms each in the SWNT (Fig. 6). The left cluster is mobile and moves along the cavity of the SWNT. The right cluster is fixed to the SWNT by a metal-carbon bond and is partially covered by a carbon shell, which can be viewed as a substrate for heterogeneous nucleation.
135 s: The left cluster shifts and adheres to the carbon shell of the right cluster.
151 s and 177 s: The two clusters come into contact twice briefly via a single Re atom
183 s: Both clusters merge within 3 s. The resulting cluster contains ~ 20 atoms. This appears to be above the critical number that allows the metal to overcome the free energy barrier to crystallization.
185 s: The crystallization process begins with the formation of a metastable amorphous state, followed by the reorganization of the Re atoms into a crystallite.
308 s: The recrystallite is observed with a recognizable crystal structure, followed by 124 s restructuring while maintaining the crystallinity, which can be clearly observed as distinguishable lattice planes in the ordered crystallite stage and quantified by FFT (Fig. 6b, c). The entire crystallite formation of Re follows the same TSNM path as Au and Fe, with the only difference being that the critical size (or number of atoms) required for crystallization is achieved by the coalescence of two amorphous Re clusters.
Three metals, three processes, one mechanism
The scientists had with this work for the first time observed the existence of a metastable amorphous precursor and its necessity for the nucleation processes. The size and number of atoms in the amorphous precursor are of crucial importance for the formation of a crystallite. For all three metals, the critical size is in the range below 2 nm and the number of atoms required for successful crystallization of γ-Fe and Re is between 10 and 20. The combined use of a SWNT as a substrate for heterogeneous nucleation and the channel for the delivery of metal atoms and the electron beam as an energy source to simultaneously power the process and act as an imaging tool, provide information about the formation of a crystal nucleus in the sub-nanometer range - a challenging size range for any other analytical method. These results are particularly important for Fe, Au, and Re in carbon-rich environments that exist in a variety of industrial contexts, including Fischer-Tropsch catalysis, growth of graphene by chemical vapor deposition, or steelmaking.
The main difference between the metals is that the heterogeneous nucleation can be triggered by various causes: the collection of individual atoms (Fe), the order of the atoms in an amorphous nanocluster (Au) or the coalescence of two separate amorphous subnanometer clusters (Re) . (Figure 3). Researchers directly observed the existence of a liquid-like metastable precursor - a fundamental component of the two-step nucleation mechanism - and identified an additional energy barrier that the amorphous precursor must overcome in order to crystallize. In addition, the atomic resolution of AC-TEM made it possible to estimate the number of atoms in the nucleating metal clusters. For all three metals, the team found that the critical size is below 2 nm and the number of atoms required for γ-Fe and Re to crystallize successfully is between 10 and 20.
Interestingly, each metal showed a different example of how the initially amorphous atomic clusters could overcome the size requirement and energy barrier for nucleation. Professor Ute Kaiser said: "By successfully recording this process, we hope that in action we will shed light on the fundamental phenomenon of nucleation and crystal growth for the three metals examined and enable a more controlled design of materials in the future."
Resource: Cao, K., Biskupek, J., Stoppiello, C.T., McSweeney, R. L., Chamberlain, T. W., Liu, Z., Suenaga, K., Skowron, S. T., Besley, E., Khlobystov, A. N., & Kaiser, U. A. Atomic mechanism of metal crystal nucleus formation in a single-walled carbon nanotube (2020) Nature chemistry, doi: 10.1038/s41557-020-0538-9, [PDF], see also the supporting information.
-
Sleutel, M., Lutsko, J., Driessche, A. E. S. V. A. N., Durán-Olivencia, M. A. & Maes, D. Observing classical nucleation theory at work by monitoring phase transitions with molecular precision. Nat. Commun. 5, 5598 (2014).
-
Habraken, W. J. E. M. et al. Ion-association complexes unite classical and non-classical theories for the biomimetic nucleation of calcium phosphate. Nat. Commun. 4, 1507 (2013).
-
Dey, A. et al. The role of prenucleation clusters in surface-induced calcium phosphate crystallization. Nat. Mater. 9, 1010–1014 (2010).
-
Erdemir, D., Lee, A. Y. & Myerson, A. S. Nucleation of crystals from solution: classical and two-step models. Acc. Chem. Res. 42, 621–629 (2009).
-
Gebauer, D. & Cölfen, H. Prenucleation clusters and non-classical nucleation. Nano Today 65, 564–584 (2011).
-
Nielsen, M. H., Aloni, S. & De Yoreo, J. J. In situ TEM imaging of CaCO3 nucleation reveals coexistence of direct and indirect pathways. Science 345, 1158–1162 (2014).
-
Gal, A. et al. Calcite crystal growth by a solid-state transformation of stabilized amorphous calcium carbonate nanospheres in a hydrogel. Angew. Chem. Int. Ed. 52, 4867–4870 (2013).
-
Navrotsky, A. Energetic clues to pathways to biomineralization: precursors, clusters and nanoparticles. Proc. Natl Acad. Sci. USA 101, 12096–12101 (2004).
-
Wolf, S. E., Leiterer, J., Kappl, M., Emmerling, F. & Tremel, W. Early homogenous amorphous precursor stages of calcium carbonate and subsequent crystal growth in levitated droplets. J. Am. Chem. Soc. 130, 12342–12347 (2008).
-
Gebauer, D., Völkel, A. & Cölfen, H. Stable prenucleation calcium carbonate clusters. Science 322, 1819–1822 (2008).
-
Baumgartner, J. et al. Nucleation and growth of magnetite from solution. Nat. Mater. 12, 310–314 (2013).
-
Tsarfati, Y. et al. Crystallization of organic molecules: nonclassical mechanism revealed by direct imaging. ACS Cent. Sci. 4, 1031–1036 (2018).
-
Bera, M. K. & Antonio, M. R. Crystallization of Keggin heteropolyanions via a two-step process in aqueous solutions. J. Am. Chem. Soc. 138, 7282–7288 (2016).
-
Lupulescu, A. I. & Rimer, J. D. In situ imaging of silicalite-1 surface growth reveals the mechanism of crystallization. Science 344, 729–732 (2014).
-
Pusey, P. N. & van Megen, W. Phase behaviour of concentrated suspensions of nearly hard colloidal spheres. Nature 320, 340–342 (1986).
-
Sosso, G. C. et al. Crystal nucleation in liquids: open questions and future challenges in molecular dynamics simulations. Chem. Rev. 116, 7078–7116 (2016).
-
Cao, K. et al. Comparison of atomic scale dynamics for the middle and late transition metal nanocatalysts. Nat. Commun. 9, 3382 (2018).
-
Khlobystov, A. N. Carbon nanotubes: from nano test tube to nano-reactor. ACS Nano 5, 9306–9312 (2011).
-
Zoberbier, T. et al. Interactions and reactions of transition metal clusters with the interior of single-walled carbon nanotubes imaged at the atomic scale. J. Am. Chem. Soc. 134, 3073–3079 (2012).
-
Loh, N. D. et al. Multistep nucleation of nanocrystals in aqueous solution. Nat. Chem. 9, 77–82 (2017).
-
Zheng, H. et al. Observation of single colloidal platinum nanocrystal growth trajectories. Science 324, 1309–1312 (2009).
-
Skowron, S. T. et al. Chemical reactions of molecules promoted and simultaneously imaged by the electron beam in transmission electron microscopy. Acc. Chem. Res. 50, 1797–1807 (2017).