Novel Inner and Outer Functionalized SWCNTs Characterized by TEM
March 15, 2024 - Despite extensive research, achieving stable functionalization of single-walled carbon nanotubes (SWCNTs) without altering their structure remains challenging. Researchers from Ulm University and the Friedrich-Alexander University Erlangen-Nuremberg have now developed a new method for simultaneous inside and outside functionalization of SWCNTs. This technique uses positively charged macrocycles to bind to the nanotubes and encapsulate polyiodide chains within, offering potential for energy storage applications.
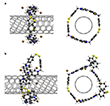
Making single-walled carbon nanotubes (SWCNTs) compatible with solution processing can be achieved through two main approaches. The first involves covalent addition reactions,1,2 which partially disrupt the sp2 framework and can affect the nanotube’s optoelectronic properties. Alternatively, non-covalent methods preserve the sp2 framework by employing weak supramolecular interactions3, biopolymer wrapping4, or detergents5. Over the past decade, researchers such as Pérez (Fig. 1a) and collaborators have expanded the applications of mechanically interlocked nanotubes (MINTs).6–10 This includes reversible disulfide exchange11–13 as a dynamic ring-closing reaction to create MINTs in organic media (Fig. 1b).14 These architectures have also been used to encapsulate dyes15 and atomic species like krypton.16 Such small-diameter peapod structures exhibit promising potential in applications ranging from conductive materials17,18 to contrast agents for biological imaging.16 In a recent study, scientists now presented a novel method for the dynamic covalent functionalization of SWCNTs (Fig. 1c) designed to ensure water solubility, synthetic accessibility, and rigidity. The interaction between macrocycles and SWCNTs is primarily driven by hydrophobic effects and van der Waals (vdW) forces.19
As illustrated in Fig. 2a, the key macrocycle was synthesized as a mixture consisting primarily of dimer (8, around 50%), trimer (9), and higher oligomers, using straightforward starting materials (1, 2, 5). This macrocycle mixture (8 and higher oligomers) was subsequently treated with the mild reducing agent dithiothreitol in a basic aqueous solution in the presence of 6,5-enriched SWCNTs. Except for the solvent, this step used functionalized SWCNTs with ex-TTF disulfide macrocycles.14 In one approach, the reaction was performed in an entirely aqueous medium adjusted to pH 9.0 (sample: MINT-1), while another used a water-DMSO mixture (1:1 v/v) at the same pH (sample: MINT-2). The resulting reaction mixtures were filtered to recover the solids, which were then washed with methanol under ultrasonication and dried under vacuum.
The researchers performed thermogravimetric analysis (TGA) of the functionalized samples (Fig. 2b) and observed a pronounced mass loss starting from 190–200 °C. In comparison, the TGA curve (blue) of the source SWCNT material showed a mass loss beginning at 400–410 °C. The mass loss in the 200–400°C range was attributed to the introduction of macrocyclic compounds during the functionalization step. The analysis revealed that MINT-1 (blue TGA curve) and MINT-2 (red TGA curve) lost 73% and 62% of their total mass in this temperature range, respectively, indicating a relatively high degree of functionalization. This high degree of functionalization was further supported by the increase in sample mass observed after thorough washing. To investigate the impact of the functionalization process, the amount of macrocycle mixture used to functionalize the SWCNT material was varied. The results showed that MINT-1 and MINT-2 were prepared with approximately 10% weight/volume of SWCNTs relative to the macrocycle mixture. A separate batch of MINT-2 was prepared with a tenfold higher fraction of SWCNTs relative to the macrocycle mixture. The corresponding TGA curves (yellow) confirmed the same mass loss pattern, with less mass lost in the 200–400°C range when the SWCNT fraction was higher.
The structure of the macrocycle, including the dimer (8), trimer (9), and higher oligomers, is shown in Figure 3a. Figure 3b presents the calculated adsorption energies for various pairs of dimeric/trimeric macrocycles interacting with SWCNTs of different chirality. To explore the topology of the new composite macrocycle/SWCNT material, aberration-corrected high-resolution transmission electron microscopy (AC-HRTEM) imaging and energy dispersive X-ray (EDX) elemental mapping were performed. In Figure 3c (center, right), sections of MINT-2 and MINT-1 are displayed, showing areas with individualized CNTs alongside typical bundled structures. Figure 3c (left) provides a detailed view of MINT-2, revealing an isolated CNT with a structure resembling a macrocycle wrapped around it. Notably, all three HRTEM images reveal chains of single atoms within the SWCNTs. Local EDX mappings (using scanning TEM mode) helped identify the distribution of carbon (red), sulfur (blue), and iodine (green) in MINT-1 (Figure 3d). The carbon distribution aligns with the HAADF (high-angle annular dark-field imaging)-STEM and BF (bright field) images of the examined samples, as expected, given that carbon is the predominant element in CNTs and the macrocyclic dimer (8), trimer (9), and oligomers. Sulfur, while more unevenly distributed, still correlates with the presence of carbon, which supports the wrapping of disulfide rings around SWCNTs. Similarly, the iodine distribution correlates with the sulfur, which could be attributed to either encapsulated polyiodide chains or externally bound counter anions.
Next, the researchers presented the calculated adsorption energies for the macrocycles with compensating Br anions, as shown in Figure 3b. They also demonstrated two corresponding structures for the (6,5)-SWCNT in Figure 4. The team found that the dispersion interaction between the macrocycles and the SWCNTs led to negative adsorption energies as long as the rings were unstrained, with the interaction scaling roughly in proportion to the ring size. However, when the diameter of the SWCNTs became too large, strain energy within the rings began to build up, resulting in a positive adsorption energy. This behavior was evident in Figure 3b for the n=2 macrocycle. Even for the thin (6,5)-SWCNT, the n=2 ring fit tightly around the nanotube (Figure 4a), though still unstrained. However, a slight increase in the SWCNT diameter was enough to induce significant strain energy (Figure 3b). In contrast, the n=3 macrocycle wrapped more loosely around all the tubes considered in the study, as evidenced by the small loops within the macrocycle (Figure 4b) or its tilted, triangular shape (Figure 5a). The researchers showed that n=2 macrocycles only formed around the thinnest SWCNTs, while rings around thicker SWCNTs consisted of three bipyridinium units. Additionally, the team found that the onset of strain energy in n=2 macrocycles, with increasing SWCNT diameter, occurred similarly when the compensating negative charge was located at or within the SWCNTs. The much lower strain energy observed was one of the key reasons the authors proposed that the AC-HRTEM image in Figure 3c depicted a trimeric macrocycle around a (10,1)-SWCNT.
The researchers used Raman spectroscopy with a 633 nm laser on a bundle of nanotubes drop-deposited onto a Si/SiO2 wafer to investigate the functionalization of single-walled carbon nanotubes (SWCNTs). The results revealed diameter-dependent radial breathing modes (RBMs), with corresponding chiral index assignments shown in Figure 6. Notably, double-walled carbon nanotubes were also observed, with the inner tubes marked by a star. The RBMs of the outer tubes were detected between 150 cm–1 and 210 cm–1, and the Raman spectrum of pure macrocycles, without SWCNTs, appeared as a dotted line. The key Raman bands were detected at approximately ~110 cm–1, ~150 cm–1, and ~225 cm–1 (Figure 6), which were attributed to triiodide chains, consistent with Raman spectra of iodide chains reported in the literature 20. The increased intensity and upshift of the peak around ~150 cm–1 suggested the formation of longer polyiodide chains inside the nanotubes, aligning with previous studies 21. Additionally, the downshift of the ~110 cm–1 Raman band, with weaker modes around ~100 cm–1, indicated that polyiodide chains are contributing to endohedral functionalization, as seen in the AC-HRTEM images (Figure 3). These findings support the important role of simultaneous endo- and exohedral functionalization in modulating the properties of SWCNTs (Figure 5).
Figure 7a presents representative Raman spectra for the untreated source material, MINT-1, and MINT-2, showing overall similar shapes, indicating that the integrity of the nanotube lattice was preserved. The spectra reveal slight upshifts in the G+, D, and 2D modes of the functionalized nanotubes, as shown in Figures 7b-d. These shifts suggest weak charge transfer interactions occurring between the components of the functionalized nanotubes, including the SWCNTs, macrocycles, and polyiodide chains. The researchers noted that the Raman and PLE analyses showed minimal influence of the endo- and exohedral functionalization on the fundamental properties of the SWCNTs. This is consistent with the noncovalent binding mode and extends the potential applications of dynamic covalent materials 23–27.
The overall comparison of the MINT-1 and MINT-2 Raman spectra reveals a stronger effect of the functionalization on the MINT-1 nanotube sample than on MINT-2, as shown in Figure 7. The D/G intensity ratio indicates an increased defect concentration for MINT-1, while the source and MINT-2 materials show similar defect concentrations, which was expected due to the noncovalent binding of the functional groups. The researchers concluded that choosing pure water for the functionalization process (MINT-1) leads to the introduction of additional defects in the carbon nanotubes. In contrast, the mixed water/DMSO solvent appears to be beneficial when a material with fewer defects is desired. This finding suggests that adjusting the solvent composition can control the degree of functionalization and defect concentration, offering valuable insights for tailoring the properties of functionalized SWCNTs.
Resource: Kraus, J., Meingast, L., Hald, J., Beil, S. B., Biskupek, J., Ritterhoff, C. L., Gsänger, S., Eisenkolb, J., Meyer, B., Kaiser, U., Maultzsch, J., von Delius, M. Simultaneous Inside and Outside Functionalization of Single-Walled Carbon Nanotubes. Angew. Chem. Int. Ed. 2024, 63, e202402417. 10.1002/anie.202402417
-
Bianco, A., & Prato, M. (2003). Can carbon nanotubes be considered useful tools for biological applications? Advanced Materials, 15(20), 1765–1768.
-
Norizan, M. N., et al. (2020). Carbon nanotubes: Functionalization and their application in chemical sensors. RSC Advances, 10(71), 43704–43732.
-
Romero-Nieto, C., et al. (2012). Tetrathiafulvalene-based nanotweezers – Noncovalent binding of carbon nanotubes in aqueous media with charge transfer implications. Journal of the American Chemical Society, 134(22), 9183–9192.
-
Gao, T. Z., et al. (2020). Engineering supramolecular polymer conformation for efficient carbon nanotube sorting. Small, 16(26), 2000923.
-
Yang, F., et al. (2020). Chirality pure carbon nanotubes: Growth, sorting, and characterization. Chemical Reviews, 120(5), 2693–2758.
-
de Juan, A., et al. (2014). Mechanically interlocked single‐wall carbon nanotubes. Angewandte Chemie International Edition, 53(21), 5394–5400.
-
Martínez-Periñán, E., et al. (2016). The mechanical bond on carbon nanotubes: Diameter-selective functionalization and effects on physical properties. Nanoscale, 8(17), 9254–9264.
-
Miki, K., et al. (2018). Unique tube–ring interactions: Complexation of single‐walled carbon nanotubes with cycloparaphenyleneacetylenes. Small, 14(26), 1800720.
-
Pérez, E. M. (2017). Putting rings around carbon nanotubes. Chemistry – A European Journal, 23(52), 12681–12689.
-
López-Moreno, A., & Pérez, E. M. (2015). Pyrene-based mechanically interlocked SWNTs. Chemical Communications, 51(25), 5421–5424.
-
Fritze, U. F., & von Delius, M. (2016). Dynamic disulfide metathesis induced by ultrasound. Chemical Communications, 52(38), 6363–6366.
-
Fritze, U. F., Craig, S. L., & von Delius, M. (2018). Disulfide‐centered poly (methyl acrylates): Four different stimuli to cleave a polymer. Journal of Polymer Science Part A: Polymer Chemistry, 56(13), 1404–1411.
-
Black, S. P., Sanders, J. K., & Stefankiewicz, A. R. (2014). Disulfide exchange: exposing supramolecular reactivity through dynamic covalent chemistry. Chemical Society Reviews, 43(6), 1861–1872.
-
Balakrishna, B., Menon, A., Cao, K., Gsänger, S., Beil, S. B., Villalva, J., Shyshov, O., Martin, O., Hirsch, A., Meyer, B., Kaiser, U., Guldi, D. M., & von Delius, M. (2020). Dynamic covalent formation of concave disulfide macrocycles mechanically interlocked with single‐walled carbon nanotubes. Angewandte Chemie International Edition, 59(42), 18774–18785.
-
Forel, S., Li, H., van Bezouw, S., Campo, J., Wieland, L., Wenseleers, W., Flavel, B. S., & Cambré, S. (2022). Diameter-dependent single-and double-file stacking of squaraine dye molecules inside chirality-sorted single-wall carbon nanotubes. Nanoscale, 14(23), 8385–8397.
-
Serpell, C. J., Rutte, R. N., Geraki, K., Pach, E., Martincic, M., Kierkowicz, M., De Munari, S., Wals, K., Raj, R., Ballesteros, B., Tobias, G., Anthony, D. C., & Davis, B. G. (2016). Carbon nanotubes allow capture of krypton, barium and lead for multichannel biological X-ray fluorescence imaging. Nature Communications, 7(1), 13118.
-
Zhao, Y., Wei, J., Vajtai, R., Ajayan, P. M., & Barrera, E. V. (2011). Iodine doped carbon nanotube cables exceeding specific electrical conductivity of metals. Scientific Reports, 1(1), 83.
-
Fujimori, T., & Urita, K. (2016). Effect of selectively intercalated polyiodide on the electric transports of metallic-and semiconducting-enriched single-wall carbon nanotube networks. Applied Physics Letters, 108(26).
-
Wang, J., Chu, H., & Li, Y. (2008). Why single-walled carbon nanotubes can be dispersed in imidazolium-based ionic liquids. ACS Nano, 2(12), 2540–2546.
-
Johnson, A. E., & Myers, A. B. (1996). Solvent effects in the Raman spectra of the triiodide ion: Observation of dynamic symmetry breaking and solvent degrees of freedom. The Journal of Physical Chemistry, 100(19), 7778–7788.
-
Yoshida, Y., Ishii, Y., Kato, N., Li, C., & Kawasaki, S. (2016). Low-temperature phase transformation accompanied with charge-transfer reaction of polyiodide ions encapsulated in single-walled carbon nanotubes. The Journal of Physical Chemistry C, 120(36), 20454–20461.
-
Hierold, C., Brand, O., Fedder, G. K., Korvink, J. G., & Tabata, O. (2008). Carbon nanotube devices: properties, modeling, integration and applications. John Wiley & Sons.
-
Geng, K., He, T., Liu, R., Dalapati, S., Tan, K. T., Li, Z., Tao, S., Gong, Y., & Jiang, D. (2020). Covalent organic frameworks: design, synthesis, and functions. Chemical Reviews, 120(16), 8814–8933.
-
Giuseppone, N. (2012). Toward self-constructing materials: a systems chemistry approach. Accounts of Chemical Research, 45(12), 2178–2188.
-
Slater, A. G., & Cooper, A. I. (2015). Function-led design of new porous materials. Science, 348(6238), aaa8075.
-
Mastalerz, M. (2010). Shape‐persistent organic cage compounds by dynamic covalent bond formation. Angewandte Chemie International Edition, 49(30), 5042–5053.
-
Haase, F., & Lotsch, B. V. (2020). Solving the COF trilemma: towards crystalline, stable and functional covalent organic frameworks. Chemical Society Reviews, 49(23), 8469–8500.