Trapped atoms form 1D gas and dimers
January 22, 2024 - Scientists have successfully trapped atoms of krypton (Kr), a noble gas, inside a carbon nanotube to form a one-dimensional gas. A team from the Universities of Nottingham, Ulm, Queensland, Southamptin, and Leeds and the SuperSTEM Laboratory have development of a molecular system for the delivery and direct observation of krypton atom dynamics in direct space and real time.
Microscopy is an important analytical tool in chemistry, as direct imaging of atoms and molecules can provide for the discovery of chemical processes at the nanoscale. Transmission electron microscopy (TEM) enables the detection of individual atom positions, with electrons acting simultaneously as an imaging probe and an energy source to drive chemical transformations in situ [1]. The combination of energy selection with high spatial and temporal resolution can facilitate the direct study of chemical processes at the atomic level, in direct space and real time. Accordingly, TEM may be used to record fundamental mechanisms, e.g., bond breaking and formation, creating opportunities to elucidate chemical processes at the single-atom level, provided a suitable encapsulating system is utilized. Investigation of atoms and molecules by TEM inside carbon nanotubes, the world’s smallest test tubes, is particularly informative, as electron beam damage to the sample system is minimized [2]. Upon coalescence of the fullerene cages, driven by the electron beam [3] or heat [4], larger molecular capsules are formed, containing combinations of individual guest atoms or molecules, the interactions between which can be studied in isolation free from the effects of external stimuli (Figure 1). The direct investigation of the bonding states of noble gas atoms is based on previous work mapping the positions of lanthanide metal atoms [5, 6]. The effect of encapsulated metal atoms within fullerenes on their coalescence rate have been studied by Koshino et al. [7]. Further, both the rate and mechanism of fullerene coalescence for the molecular endohedral species have been studied by Biskupek et al. [8]. However, the controlled use of energy transfer in TEM for the coalescence of endohedral fullerenes is based on ChemTEM [1, 9] and related studies [10] that combine energy transfer to trigger chemical reactions and to image chemical processes at the molecular level in situ in high-resolution TEM films. The systems investigated using this technique include: (i) polyoxometalates (POMs) [11]; (ii) perchlorocoronene (PCC) [12]; (iii) metal halide nanoclusters [13]; (iv) C60 [14]; and (v) diatomic metal clusters [15].
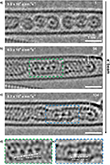
TEM is applied to investigate the atomic-scale dynamics and bonding of the noble gas krypton. Previous investigations of krypton atoms by microscopy involved entrapment of Kr by ion implantation in bilayer graphene [16, 17]. Figure 2a is an aberration-corrected high-resolution TEM (AC-HRTEM) image, recorded at 80 kV, showing the structure of (Kr@C60)@SWCNT. Individual endohedral Kr atoms exhibit strong contrast at the center of each C60 molecule. High Kr@C60 purity is confirmed by TEM images, where nearly all the C60 cages were filled. Each Kr atom lies close to the geometric center of its host C60 cage. The close match in size between the van der Waals diameter of Kr (0.404 nm) [18] and the internal cavity of C60 (∼0.4 nm) (Figure 2b) results in symmetrical electronic repulsion, forcing the Kr nucleus to the center, in turn leading to enhanced Kr atom definition due to dampened atomic vibration, reducing motion blur within the cage. Kr atomic position and identity were also confirmed by aberration-corrected high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM) and electron energy loss spectroscopy (EELS). Figure 2c,d presents HAADF-STEM images of (Kr@C60)@SWCNT recorded at 60 kV to minimize beam-induced damage, hence aiding native state preservation. In addition, Figure 2e−g present STEM-EELS mapping of the area of (Kr@C60)@SWCNT displayed in Figure 2d, showing (Figure 2e) C K-edge and (Figure 2f) Kr M-edge regions, respectively, and (Figure 2g) a false color composite C/Kr map, illustrating unambiguously the presence of individual Kr atoms within each C60 cage in this area. Figure 2h shows the corresponding EEL spectrum (integrated over the area highlighted by the green box in Figure 2g), with the Kr M4,5-edge onset at 89 eV.
Figure 3a presents a HAADF-STEM image of a bundle of (nKr@C60n)@SWCNT recorded at 60 kV, where mobile Kr atoms are identified as a continuous line of increased brightness in the center of the nested nanotube in the right-hand and bottom half of the left-hand CNT. Highly mobile Kr atom visibility in HAADF-STEM was attributed to the combination of single-atom brightness approximately proportional to Z2 and fast scan rate (μs dwell time per pixel). Figure 3b−d presents STEM-EELS mapping of the same area as in Figure 3a, showing (Figure 3b) C K-edge and (Figure 3c) Kr M-edge regions, respectively, and (Figure 3d) a false color composite C/Kr map, confirming the retention and identity of mobile Kr atoms free to translate post thermal coalescence of fullerene cages, behaving as a 1D gas. Figure 3e shows the corresponding EEL spectrum (integrated over the area in the green box in Figure 3d), with KrM4,5-edge onset at 89 eV. Figure 3f illustrates a long, well-annealed section of a nested nanotube, where mobile Kr atoms fill almost the entire length, but a defect forms a bottleneck past which Kr cannot transit (arrowed), as the van der Waals diameter of Kr fills entirely the 0.7 nm diameter of the annealed nanotube. Figure 3g shows an area with several intact Kr@C60 molecules on the left-hand, and a more defective nested nanotube on the right-hand. It should be noted, however, that the presence of completely delocalized, freely translating Kr atoms within the nested nanotube, providing the physical realization of one-dimensional gas models hypothesized in numerous theoretical studies, in principle giving insights into physical phenomena such as heat conduction and diffusion or hydrodynamics [19−21]. Interestingly, the associations of Kr atoms are still too mobile in the absence of a fixation point, which leads to a contrast blurring of individual atoms in HAADF-STEM images [22]. It is still unclear whether such fast-dynamic atoms move as connected short chains or as individual atoms and which states they assume. However, the use of electron cameras with higher frame rates in combination with low temperatures could solve this challenge in the future.
Figure 4a−d presents a representative selection of time-series images charting the latter stages of coalescence of adjacent Kr@C60 molecules, recorded at 80 kV under a constant electron flux of 1 × 107 e−nm−2 s−1. Indeed, due to the stochastic nature of electron-beam-induced reactions, a 2Kr@C120 bottleneck “peanut” intermediate was imaged at the start of this series, formed during the search and focusing stage before time-series acquisition (Figure 4a,e). The peanut annealed with time to form a C120 nanotube-type capsule, allowing the free interaction of guest Kr atoms in one dimension, following which Kr···Kr separations (dKr−Kr) could be determined via intensity profiling and attributed to particular Kr2 bonding states. Separations corresponding to Figure 4a−d are highlighted, emphasizing the variation in Kr2 bonding states. This plot indicates three distinct regimes corresponding to the separation of Kr···Kr while experiencing constriction, followed by heavily damped free translation as the Kr atoms were released, and then closer Kr bonding as a more settled, stable configuration became established. During Kr restriction by the C120 peanut bottleneck (Regime I), the Kr···Kr separation was found to decrease continuously, initially from 0.70 to 0.53 nm (Figure 4a) as the e-beam drove the widening of the peanut (2Kr)@C120 bottleneck. With increasing fluence, the bottleneck widened sufficiently to form a nested (2Kr)@C120 nanocapsule structure with a rapid decrease in dKr−Kr from 0.64 to 0.37 nm in ∼8 s, indicating a sharp transition toward free Kr atom translation along the nanotube axis (Regime II). For example, representative images in Figure 4b,c illustrate distinct separations of 0.37, 0.61, and 0.40 nm, respectively.
Kr atoms encapsulated within such C120 nanocapsules showed increased motion during image acquisition when compared to Kr@C60, as evidenced by noncircular atomic contrast (e.g. Figure 4c,d) representative of the weighted average of atom positions during exposure. The difference in dKr−Kr could be as large as 0.1 nm, effectively highlighting the short lifetimes of <0.4 nm separations. Hence, the measurement of dKr−Kr via intensity profiling represents the average separation captured during exposure. Nevertheless, careful observation demonstrated distinct Kr−Kr separations alternating between extremes of ∼0.6 and ∼0.4 nm, respectively, in the manner of a highly damped oscillation, consistent with distinct states attributable to nonbonded Kr···Kr and a van der Waals Kr2 dimer, respectively (Figure 4f,g). Eventually, the Kr atom pair equilibrated (Regime III) to the van der Waals bonded dimer separation, while occasionally decreasing to between 0.32 and 0.38 nm, indicative of stronger Kr−Kr bonding (Figure 4d,h). Figure 4b,f revealed slight distortion to the C120 nanocapsule (arrowed), consistent with the theoretical suggestion that a perfect (5,5) C120 nanotube is not necessarily formed [23], while noting the capsule, in this state, did not inhibit Kr atom bonding.
It is noted that the close-packed atomic spacing of Kr in face-centered cubic (fcc) crystallites was previously determined to be 0.399 nm by X-ray diffractometry [24]. This is commensurate with the van der Waals Kr2 dimer separation observed directly here by TEM after stabilization of Kr atom pairs (Regime III; Figure 4c,g), being the favored configuration distinct from non-interacting gaseous species. Several instances of Kr−Kr atom separation significantly below 0.4 nm were observed during Regime III, e.g., down to 0.33 nm. The lifetime of each of these separations was again at least on the scale of the 0.5 s exposure time for data acquisition, i.e., much longer than expected for a transient minimum for a neutral van der Waals dimer where strong repulsion due to the Pauli exclusion principle would act quickly to re-establish the energetically favored 0.4 nm Kr atom separation. Hence, the presence of relatively long-lived, <0.4 nm Kr−Kr separations is consistent with the formation of a transient covalent bond, i.e., in the form of a cationic dimer [Kr2]+.
Figure 5a−c presents a time-lapse series from a short region of partially thermally coalesced Kr@C60 molecules where six Kr atoms remained distinct, as the electron beam annealed nested nanotube structural defects, thus removing barriers to Kr translation. In particular, Figure 5b shows a chain of four interacting Kr atoms, pinned to the left-hand side of the nanotube, with dKr−Kr spacings of 0.43, 0.38, and 0.38 nm respectively (Figure 8d), indicative of a pinned terminal Kr atom attached to a Kr3 trimer, along with an isolated Kr atom midtube and another pinned terminal Kr atom at the other end (arrowed). Continued observation (Figure 5c) provided a snapshot of the translation of the Kr3 trimer, now midtube, with spacings of 0.43 and 0.40 nm, respectively (Figure 5e), closer to the favored van der Waals separation, with the terminal Kr atoms remaining pinned (arrowed) and the sixth atom moving too quickly to be imaged. It is considered that the higher surface area associated with the curvature of the end caps contributes to the pinning of terminal Kr atoms.
Resource: Cardillo-Zallo, I., Biskupek, J., Bloodworth, S., Marsden, E. S., Fay, M. W., Ramasse, Q. M., Rance, G. A., Stoppiello, C. T., Cull, W. J., Weare, B. L., Whitby, R. J., Kaiser, U., Brown, P. D., & Khlobystov, A. N. (2024). Atomic-Scale Time-Resolved Imaging of Krypton Dimers, Chains and Transition to a One-Dimensional Gas. ACS Nano, 18, 2958−2971.
-
Skowron, S. T., Chamberlain, T. W., Biskupek, J., Kaiser, U., Besley, E., & Khlobystov, A. N. (2017). Chemical reactions of molecules promoted and simultaneously imaged by the electron beam in transmission electron microscopy. Accounts of Chemical Research, 50(8), 1797-1807.
-
Khlobystov, A. N. (2011). Carbon nanotubes: from nano test tube to nano-reactor. ACS Nano, 5(12), 9306-9312.
-
Hernandez, E., Meunier, V., Smith, B. W., Rurali, R., Terrones, H., Buongiorno Nardelli, M., Terrones, M., Luzzi, D. E., & Charlier, J. C. (2003). Fullerene coalescence in nanopeapods: a path to novel tubular carbon. Nano Letters, 3(8), 1037-1042.
-
Smith, B. W., & Luzzi, D. E. (2000). Formation mechanism of fullerene peapods and coaxial tubes: a path to large scale synthesis. Chemical Physics Letters, 321(1-2), 169-174.
-
Chuvilin, A., Khlobystov, A. N., Obergfell, D., Haluska, M., Yang, S., Roth, S., & Kaiser, U. (2010). Observations of chemical reactions at the atomic scale: dynamics of metal-mediated fullerene coalescence and nanotube rupture. Angew. Chem., Int. Ed, 49(1), 193-196.
-
Warner, J. H., Ito, Y., Rümmeli, M. H., Gemming, T., Büchner, B., Shinohara, F. H., & Briggs, G. A. D. (2009). One-dimensional confined motion of single metal atoms inside double-walled carbon nanotubes. Physical Review Letters, 102(19), 195504.
-
Koshino, M., Niimi, Y., Nakamura, E., Kataura, H., Okazaki, T., Suenaga, K., & Iijima, S. (2010). Analysis of the reactivity and selectivity of fullerene dimerization reactions at the atomic level. Nature Chemistry, 2(2), 117-124.
-
Biskupek, J., Skowron, S. T., Stoppiello, C. T., Rance, G. A., Alom, S., Fung, K. L., Whitby, R. J., Levitt, M. H., Ramasse, Q. M., Kaiser, U., Besley, U., & Khlobystov, A. N. (2020). Bond dissociation and reactivity of HF and H2O in a nano test tube. ACS Nano, 14(9), 11178-11189.
-
Chamberlain, T. W., Biskupek, J., Skowron, S. T., Markevich, A. V., Kurasch, S., Reimer, O., Walker, K. E., Rance, G. A., Feng, X., Müllen, K., Turchanin, A., Lebedeva, M. A., Majouga, A. G., Nenajdenko, V. G., Kaiser, U., Besley, E., & Khlobystov, A. N. (2017). Stop-frame filming and discovery of reactions at the single-molecule level by transmission electron microscopy. ACS Nano, 11(3), 2509-2520.
-
Nakamura, E. (2017). Atomic-resolution transmission electron microscopic movies for study of organic molecules, assemblies, and reactions: The first 10 years of development. Accounts of Chemical Research, 50(6), 1281-1292.
-
Jordan, J. W., Fung, K. L., Skowron, S. T., Allen, C. S., Biskupek, J., Newton, G. N., Kaiser, U., & Khlobystov, A. N. (2021). Single-molecule imaging and kinetic analysis of intermolecular polyoxometalate reactions. Chemical Science, 12(21), 7377-7387.
-
Fung, K. L., Skowron, S. T., Hayter, R., Mason, S. E., Weare, B. L., Besley, N. A., Ramasse, Q. M., Allen, C. S., & Khlobystov, A. N. (2023). Direct measurement of single-molecule dynamics and reaction kinetics in confinement using time-resolved transmission electron microscopy. Physical Chemistry Chemical Physics, 25(13), 9092-9103.
-
Botos, A., Biskupek, J., Chamberlain, T. W., Rance, G. A., Stoppiello, C. T., Sloan, J., Liu, Z., Suenaga, K., Kaiser, U., & Khlobystov, A. N. (2016). Carbon nanotubes as electrically active nanoreactors for multi-step inorganic synthesis: sequential transformations of molecules to nanoclusters and nanoclusters to nanoribbons. Journal of the American Chemical Society, 138(26), 8175-8183.
-
Okada, S., Kowashi, S., Schweighauser, L., Yamanouchi, K., Harano, K., & Nakamura, E. (2017). Direct microscopic analysis of individual C60 dimerization events: Kinetics and mechanisms. Journal of the American Chemical Society, 139(50), 18281-18287.
-
Cao, K., Skowron, S. T., Biskupek, J., Stoppiello, C. T., Leist, C., Besley, E., Khlobystov, A. N., & Kaiser, U. (2020). Imaging an unsupported metal–metal bond in dirhenium molecules at the atomic scale. Science Advances, 6(3), eaay5849.
-
Längle, M., Mizohata, K., Åhlgren, E. H., Trentino, A., Mustonen, K., & Kotakoski, J. (2020). 2D Noble Gas Crystals Encapsulated in Few-Layer Graphene. Microscopy and Microanalysis, 26(S2), 1086-1089.
-
Längle, M., Mizohata, K., Mangler, C., Trentino, A., Mustonen, K., Åhlgren, E. H., & Kotakoski, J. (2024). Two-dimensional few-atom noble gas clusters in a graphene sandwich. Nature Materials, 23(6), 762-767.
-
Bondi, A. V. (1964). van der Waals Volumes and Radii. The Journal of Physical Chemistry, 68(3), 441-451.
-
Casati, G. (2005). Controlling the heat flow: Now it is possible. Chaos: An Interdisciplinary Journal of Nonlinear Science, 15(1).
-
Li, B., Wang, J., Wang, L., & Zhang, G. (2005). Anomalous heat conduction and anomalous diffusion in nonlinear lattices, single walled nanotubes, and billiard gas channels. Chaos: An Interdisciplinary Journal of Nonlinear Science, 15(1).
-
Chakraborti, S., Ganapa, S., Krapivsky, P. L., & Dhar, A. (2021). Blast in a one-dimensional cold gas: from Newtonian dynamics to hydrodynamics. Physical Review Letters, 126(24), 244503.
-
Cull, W. J., Skowron, S. T., Hayter, R., Stoppiello, C. T., Rance, G. A., Biskupek, J., Kudrynskyi, Z. R., Kovalyuk, Z. D., Allen, C. S., Slater, T. J. A., Kaiser, U., Patané, A., & Khlobystov, A. N. (2023). Subnanometer-wide indium selenide nanoribbons. ACS Nano, 17(6), 6062-6072.
-
Simon, F., & Monthioux, M. (2011). Fullerenes inside carbon nanotubes: the peapods. Carbon Meta‐Nanotubes: Synthesis, Properties and Applications, 273-321.
-
Sonnenblick, Y., Alexander, E., Kalman, Z. H., & Steinberger, I. T. (1977). Hexagonal close packed krypton and xenon. Chemical Physics Letters, 52(2), 276-278.